Machine vision inspection of capsule appearance is a crucial aspect of quality control in the pharmaceutical industry. It can accurately detect the appearance of capsules and quickly identify any issues with size, color, marking, etc. Compared to manual inspection, the machine vision system has a higher inspection speed and accuracy, significantly improving production efficiency and product quality. At the same time, it can continuously monitor and record inspection data, which can help identify the source of the problem and optimize the production process. It can be said that machine vision inspection of capsule appearance is an important guarantee for pharmaceutical companies to improve product quality and reduce risks, and plays an essential role in ensuring medical safety and enhancing market competitiveness.
The following case of capsule defect detection design thought is shared by Intsoft Tech.
Working principle of the machine
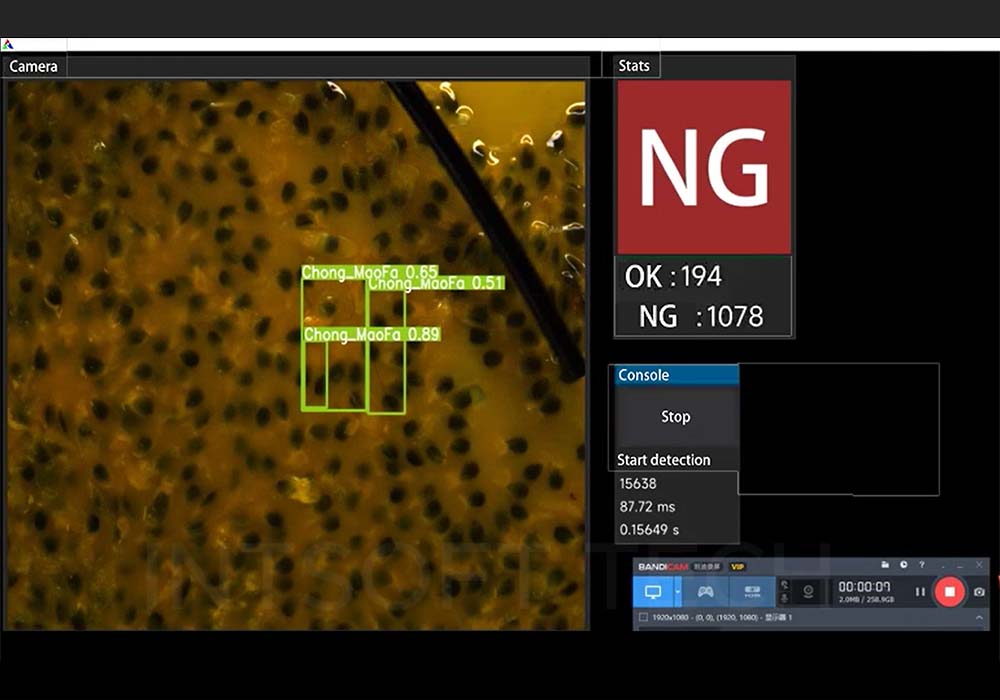
Pour the capsules to be tested into the drop port. When the capsules are placed on the turntable from the inlet, the turntable is responsible for separating the capsules one by one and placing them in the capsule slot. At this time, the conveyor chain is in a state of continuous rotation. Since the photoelectric sensor will detect the rotation of the conveyor chain, the light source will flash the capsule as it passes the designated conveying position. At this time, the industrial camera will also start capturing the capsule image and transfer the collected image data to the PC’s memory. The capsule detection system installed on the PC will determine capsule image defects. When the capsule is judged to be defective, an air punch signal is output to place the defective capsule in the unqualified capsule box, and the capsule that passes the inspection will enter the qualified box.
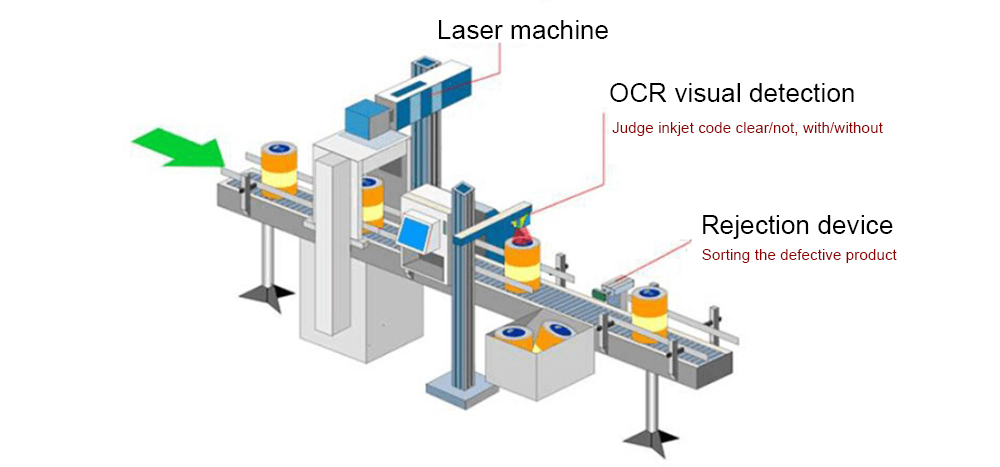
Different acquisition schemes are required for different defect characteristics of capsules. The capsule inspection software is responsible for controlling the operation process of the whole system, such as adjusting the light source, setting camera parameters, setting capsule image configuration parameters and controlling other software. Its performance is directly related to the success or failure of the entire visual inspection system.
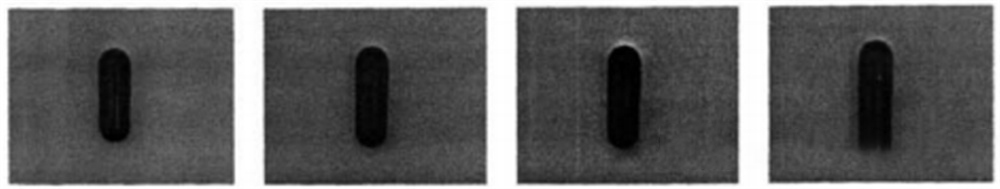
Process
In the whole process of image processing, first of all through the image sensor CCD image sensor to obtain the image, and the image converted to a computer or microprocessor can recognize and run the digital signal, so that through the A/D converter will be converted to the capsule image into a digital image. Acquisition of image after capsule preprocessing (filtering, image enhancement, etc.), extraction of capsule image features, classification and identification.
For unqualified capsules detected, the detection system sends a signal to the PLC indicating that a defective capsule has been detected, and the reject relay needs to work to put the unqualified product into the scrap chute.
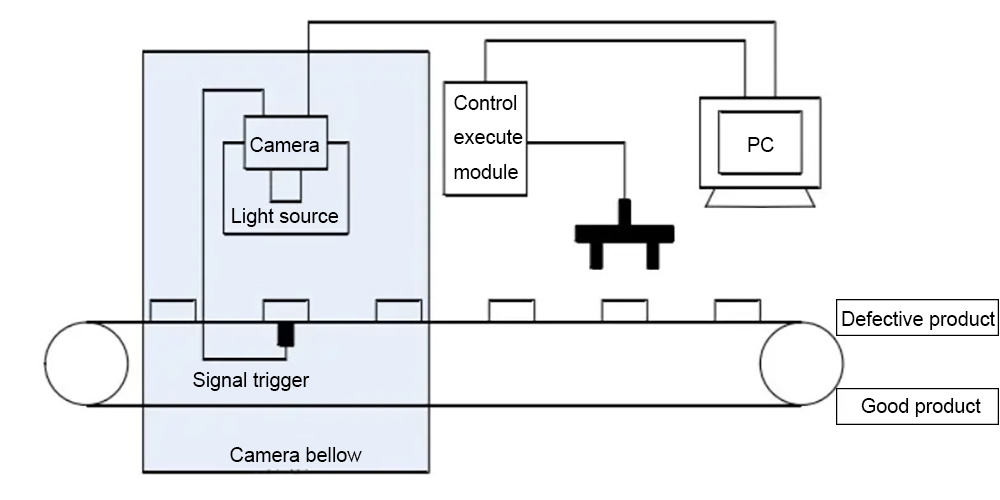
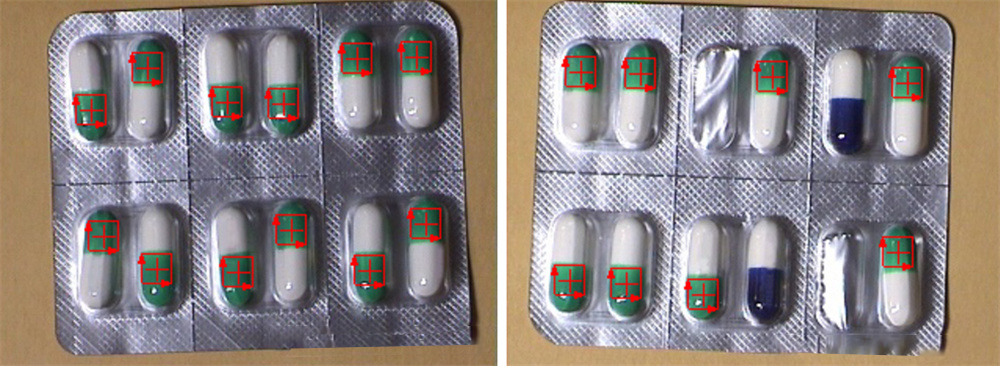
The image below shows the three locations where the capsule was detected:
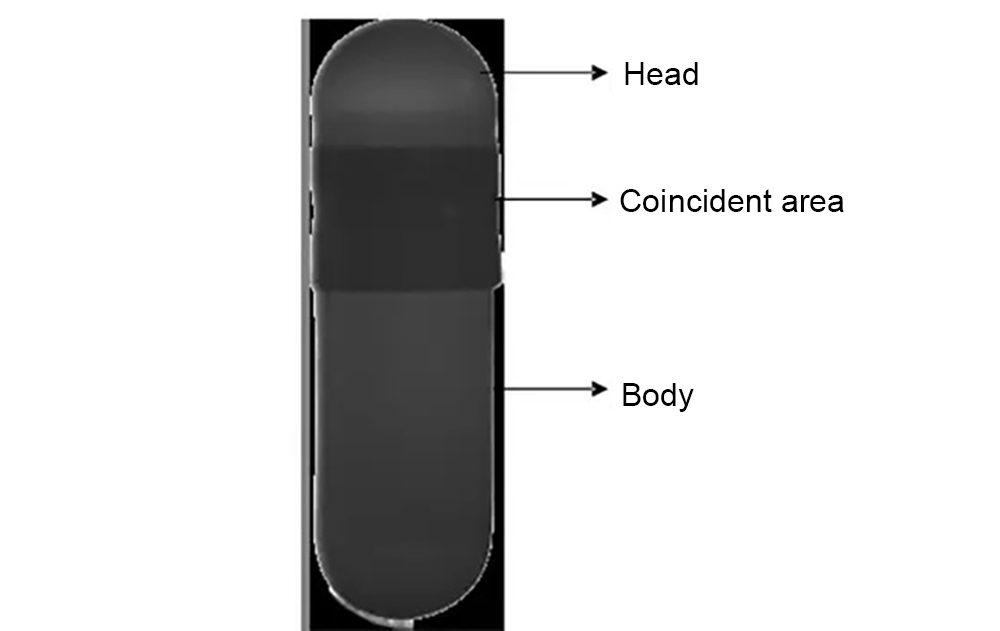
Intsoft Tech’s algorithm analysis
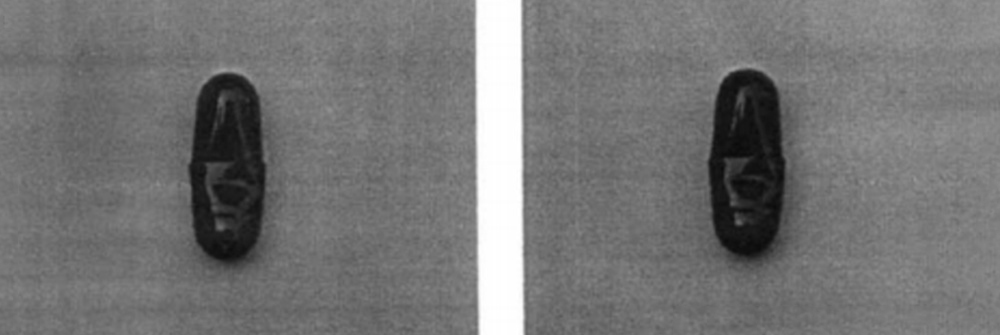
After the capsule is filled by the filling machine, the capsule body and capsule cap are well matched. During the inspection process, the capsule may be damaged or the surface worn due to human factors, machine failure, stacking and other factors. Depending on the degree of damage and wear, the system must remove capsules with severe damage or wear. Those damaged or worn capsules with minor damage or wear that does not affect the effect of capsule use may be considered qualified capsules to reduce losses in the production process.